All India Engineering / Architecture Entrance Examination (AIEEE)
CBSE Guess > AIEEE > AIEEE Papers > 2009 > Chemistry
AIEEE 2009 Chemistry
Q. 15. The two functional groups present in a typical carbohydrate are:
- –OH and –CHO
- –OH and –COOH
- –CHO and –COOH
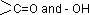
Sol.: Carbohydrate is a polyhydroxy aldehyde or polyhydroxy ketone or any compound which give these on hydrolysis.  group includes aldehydic as well as ketonic group.
group includes aldehydic as well as ketonic group.
Answer : (4)
Q. 16. Calculate the wavelength (in nanometer) associated with a proton moving at ![]()
- 14.0 nm
- 0.032 nm
- 0.40 nm
- 2.5 nm
Sol:
![]()
Answer: (3)
Q. 17. The bond dissociation energy of B–F in BF3 is 646 kJ mol–1 whereas that of C–F in CF4 is 515 kJ mol–1. The correct reason for higher B–F bond dissociation energy as compared to that of C–F is
- lower degree of
 interaction between B and F in BF3than that between C and F in CF4.
interaction between B and F in BF3than that between C and F in CF4.
- smaller size of B atoms as compared to that of C atom.
- stronger
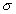 bond between B and F in BF3 as compared to that between C and F in CF4
bond between B and F in BF3 as compared to that between C and F in CF4
- significant
 interaction between B and F in BF3 whereas there is no possibility of such interaction between C and F in CF4 .
interaction between B and F in BF3 whereas there is no possibility of such interaction between C and F in CF4 .
Sol: Boron in BF3 has a vacant p–orbital, allowing ![]() back bonding while carbon in CF4 has no vacant orbital, so no back bonding is feasible. Thus, B–F bond is stronger than CF4.
back bonding while carbon in CF4 has no vacant orbital, so no back bonding is feasible. Thus, B–F bond is stronger than CF4.
Answer: (4)