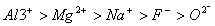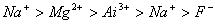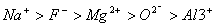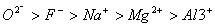All India Engineering / Architecture Entrance Examination (AIEEE)
CBSE Guess > AIEEE > AIEEE Papers > 2010 > Chemistry
AIEEE 2010 Chemistry
Q. 21. The correct sequence which shows decreasing order of the ionic radii of the elements is
So l :
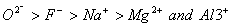 are isolectronic species having 10 electrons each. Cation having positive charge is smallest in size and the anion with high negative charge is largest in size.
are isolectronic species having 10 electrons each. Cation having positive charge is smallest in size and the anion with high negative charge is largest in size.
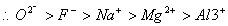
Answer: (4)
Q. 22. Percentages of free space in cubic close packed structure and in body centered packed structure are respectively
- 30% and 26%
- 26% and 32%
- 32% and 48%
- 48% and 26%
Sol :
Packing efficiency of CCP and BCC structures are 74% and 68% respectively, so the % of free space in CCP and BCC are 26% and 32% respectively.
Answer: (2)
Q. 23. In aqueous solution the ionization constants for carbonic acid are
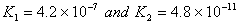
Select the correct statement for a saturated 0.034 M solution of the carbonic acid.
- The concentration of
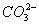 is 0.034 M.
is 0.034 M. - The concentration of
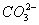 is greater than that of
is greater than that of 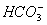
- The concentrations of
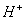 and
and 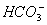 are approximately equal.
are approximately equal. - The concentration of
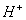 is double that of
is double that of 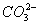 .
.
Sol :
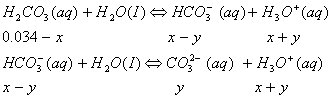
The 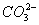 concentration cannot be 0.034 as H2CO3 and
concentration cannot be 0.034 as H2CO3 and 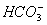 are weak acids.
are weak acids. 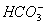 dissociates very little in second step, such that
dissociates very little in second step, such that 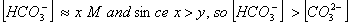 . Concentration of
. Concentration of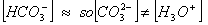
Answer: (3)