All India Engineering / Architecture Entrance Examination (AIEEE)
CBSE Guess > AIEEE > AIEEE Papers > 2010 > Chemistry
AIEEE 2010 Chemistry
Q. 28. 29.5 mg of an organic compound containing nitrogen was digested according to Kjeldahl’s method and the evolved ammonia was absorbed in 20 mL of 0.1 M HCl solution. The excess of the acid required 15 mL of 0.1 M NaOH solution for complete neutralization. The percentage of nitrogen in the compound is
- 59.0
- 47.4
- 23.7
- 29.5
Sol :
Mass of organic compound 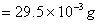
Initial moles of HCl taken 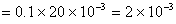
Moles of NaOH reacted with excess of HCl 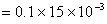
Moles of excess of HCl 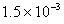
Moles of HCl that reacted with 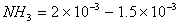
Moles of NH3 absorbed 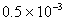
Moles of N in NH3 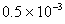
Mass of N in organic compound 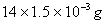
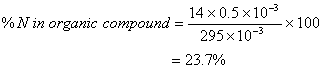
Answer:
(3)
Q. 29. Consider the reaction:
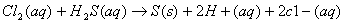
The rate equation for this reaction is s
Rate = k[Cl2] [H2S]
Which of these mechanisms is/are consistent with this rate equation?
(A)
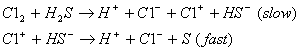
(B)
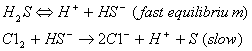
- (B) only
- Both (A) and (B)
- Neither (A) nor (B)
- (A) only
Sol : According to mechanism (A) the rate law expression is
Answer:
4
Q. 30. One mole of a symmetrical alkene on ozonolysis gives two moles of an aldehyde having a molecular mass of 44 u. The alkene is.
- propene
- 1-butene
- 2-butene
- ethene
Sol :
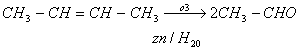
Molecular mass of CH3CHO is 44 u.
Answer: (3)