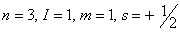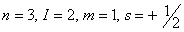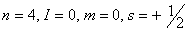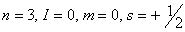All India Engineering / Architecture Entrance Examination (AIEEE)
CBSE Guess > AIEEE > AIEEE Papers > 2007 > Chemistry
AIEEE 2007 Chemistry
Q. 23. Which of the following sets of quantum numbers represents the highest energy of an atom?
Sol: An orbital having higher value of (n + l) has higher energy. Out of the four options given, the value of (n + l) is highest if n = 3 and l = 2. Correct choice: (2)
Q. 24. Which of the following hydrogen bonds is the strongest?
- O–H.…N
- F–H.…F
- O–H.…O
- O–H.…F
Sol: Greater the difference in electronegativity of H–atom and the other electronegative atom with which H is covalently bonded, stronger is the hydrogen bond. Highest electronegativity difference exists in HF molecule. Correct choice: (2)
Q. 25. In the reaction, ![]()
- 6 L HCl(aq) is consumed for every 3 L H2(g) produced.
- 33.6 L H2(g) is produced regardless of temperature and pressure for every mole Al that reacts.
- 67.2 L H2(g) at STP is produced for every mole Al that reacts.
- 11.2 L H2(g) at STP is produced for every mole HCl(aq) consumed.
Sol: Given that, ![]()
For each mole of Al reacted, 1.5 mol of H2 is formed; and for each mole of HCl(aq) consumed, 0.5 mol or 11.2 L of H2 at STP is formed.
Correct choice: (4)
Q. 26. Regular use of which of the following fertilizers increases the acidity of soil?
- Potassium nitrate
- Urea
- Superphosphate of lime
- Ammonium sulphate
Sol: Ammonium sulphate is a salt of weak base and strong acid. Its aqueous solution is acidic due to hydrolysis of ammonium ion. It will increase the acidity of soil. Correct choice: (4)
Q. 27. Identify the correct statement regarding a spontaneous process:
- For a spontaneous process in an isolated system, the change in entropy is positive.
- Endothermic processes are never spontaneous.
- Exothermic processes are always spontaneous.
- Lowering of energy in the reaction process is the only criterion for spontaneity.
Sol: For a process to be spontaneous in an isolated system, the change in entropy is positive. Correct choice: (1)