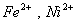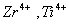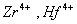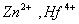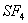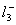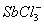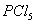AIPMT 2010 Solved Papers
AIPMT Examination
AIPMT Solved / Unsolved Papers
AIPMT Important Links
AIPMT 2010 Chemistry
Q. 31. Which of the following pairs has the same size?
Sol:
ue to lanthanide contraction, the size of Zr and Hf (atom and ions) remain constant
Answer : (3)
Q. 32. In which one of the following species the central atom has the type of
hybridization which is not the same as that present in the other three?
Sol:
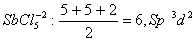
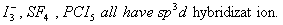
Answer : (3)
Q. 33. Which of the following represents the correct order of increasing electron gain enthalpy with negative sign for the elements O, S, F and Cl?
- Cl < F < O < S
- O < S < F < Cl
- F < S < O < Cl
- S < O < Cl < F
Sol:
O < S < F < Cl
Electron gain enthalpy − 141 − 200 − 333 − 349 kJ mol−1
Answer : (2)