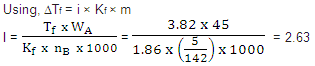AIPMT Examination
AIPMT Solved / Unsolved Papers
AIPMT Important Links
Chemistry 2011
Q. 11. The van't Hoff factor i for a compound which undergoes dissociation in one solvent and association in other solvent is respectively
(1) Greater than one and greater than one
(2) Less than one and greater than one
(3) Less than one and less than one
(4) Greater than one and less than one
Sol .
Fact
Answer : (4)
Q. 12. Standard electrode potential for ![]() couple is +0.15 V and that for the
couple is +0.15 V and that for the ![]() couple is –0.74 V. These two couples in their standard state are connected to make a cell. The cell potential will be
couple is –0.74 V. These two couples in their standard state are connected to make a cell. The cell potential will be
(1) +1.83 V (2) +1.19 V
(3) +0.89 V (4) +0.18 V
Sol:
Answer : (3)
Q.13. A gaseous mixture was prepared by taking equal mole of CO and N2. If the total pressure of the mixture was found 1 atmosphere, the partial pressure of the nitrogen (N2) in the mixture is
(1) 1 atm (2) 0.5 atm
(3) 0.8 atm (4) 0.9 atm
Sol.
Fact
Answer : (2)
Q. 14. If the ![]() for a given reaction has a negative value, then which of the following gives the correct relationships for the values of ΔG° and Keq?
for a given reaction has a negative value, then which of the following gives the correct relationships for the values of ΔG° and Keq?
Sol:
Answer : (1)
Q. 15. The freezing point depression constant for water is –1.86° cm -1 If 5.00 g Na2SO4 is dissolved in 45.0 g H2O,the freezing point is changed by –3.82°C. Calculate the van't Hoff factor for Na2SO4
(1) 0.381 (2) 2.05
(3) 2.63 (4) 3.11
Sol:
Answer : (3)