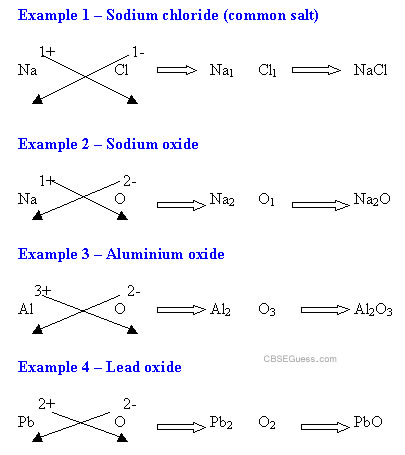
PREFIX OR
SUFFIX |
MEANING |
EXAMPLE |
Mono- |
There is 1 atom of that type in that molecule |
Carbon monoxide (CO) |
Di- |
There are 2 atoms of that type in the molecule |
Carbon dioxide (CO2) |
Bi- |
Hydrogen is present in the molecule |
Sodium bicarbonate
(NaHCO3) |
-ide |
There are only 2 types of atoms present in the molecule |
Lead oxide
(PbO) |
-ate |
There are 3 or more types of atoms in the molecule, and 1 type is oxygen |
Calcium carbonate
(CaCO3) |