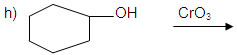![]()
CBSE Guess > Papers > Important Questions > Class XII > 2013 > Chemistry > Aldehyde,Ketone and Carboxylic acid by Mr. R. Srinivas Vasudevamurthy
CBSE CLASS XII
Aldehyde,Ketone and Carboxylic acid - 6 Marks Questions
1. Write equations for the following named reactions:
a) Nucleophillic addition reaction.
b) clemensen reduction.
c) wolf kishner reduction.
d) aldol condensation.
e) crossed aldol condensation
f) cannizaro reaction.
g) Rosenmund's reduction.
h) kolbe's electrolysis
i) Stephen reaction.
j) Etard reaction.
k) decarboxylation.
l) acylation.
m) schotten baumann reaction.
n) Friediel craft's acylation.
o) esterification.
p) Gattermann- Koch reaction.
q) Hell Volhard Zelensky reaction.
2. Explain
a) Fehling's test
b) Tollen's reagent test with suitable examples.
3. Distinguish chemically between the following pairs of organic compounds:
a) Methanal and ethanal.
b) ethanal and propanone.
c) pentan-2-one and pentan-3-one.
d) aceto phenone and benzo phenone.
e) formic acid and acetic acid.
f) benzoic acid and ethyl benzoate.
g) phenol and benzoic acid.
h) ethanal and propanal.
i) propanal and diethyl ether.
j) benzaldehyde and acetophenone.
k) ethanal and propanone
4. Account for the following:
a) Aldehyde and ketone are polar in nature.
b) Aldehyde and ketones have higher boiling point than hydro carbons of comparable molar mass.
c) Aldehyde and ketones have lower boiling point than alcohols of comparable molar mass.
d) Ketone has higher boiling point than aldehyde of comparable molar mass.
e) Oxidation of primary alcohol to aldehyde is carried out using PCC as an oxidizing agent.
f) Rosenmund's reduction of acid chloride to aldehyde is carried out using quinoline and sulphur.
g) Aldehyde is more reactive than ketone towards nucleophile.
h) Butanone is less reactive than propanone.
i) 2,2,6-Tri methyl cyclo hexanone is less reactive towards nucleophile than cyclo hexanone.
j) Para nitro benzaldehyde is more reactive towards nucleophile than benzaldehyde.
k) Para methyl benzaldehyde is less reactive towards nucleophile than benzaldehyde.
m) Reaction of aldehyde with alcohol to give acetal is carried out in the presence of HCl(g).
n) Formaldehyde and benzaldehyde undergoes cannizaro reaction and not aldol condensation.
o) Acetaldehyde undergoes aldol condensation and not cannizaro reaction.
p) Aromatic aldehyde and ketones undergoes electrophillic substitution at meta position.
q) Carboxylic acid do not show the reactions of aldehyde and ketone though it has >C=O group.
r) Carboxylic acid has higher boilimg point than aldehyde, ketone and alcohol of comparable molar mass.
s) In semi carbazide, only one NH2 group is involved in the formation of semi carbazone.
t) Aldehyde, ketone and carboxylic acid are soluble in water.
u) In oxidation of primary alcohol to carboxylic acid is not carried out using acidified potassium dichromate.
v) Carboxylic acid is more acidic than alcohol.
w) Carboxylic acid is more acidic than phenol.
x) Acidity of CCl3COOH>CHCl2COOH>CH2ClCOOH>CH3COOH.
y) Acidity of FCH2COOH>ClCH2COOH>BrCH2COOH>I CH2COOH.
z) a chloro propanoic acid is more acidic than � chloro propanoic acid.
aa) Acetic acid is less acidic than formic acid.
bb) Pure acid halide is prepared by the reaction of carboxylic acid with thionyl chloride.
cc) Carbon in carbonyl group of aldehyde and ketone acts as Lewis acid(electrophile) while oxygen acts as Lewis base ( nucleophile).
dd) Benzoic acid does not undergo Friediel craft alkylation reaction.
5. How is
a) HCHO
b) CH3CHO
c) C6H5CHO prepared commercially? Mention two uses of each.
6. How is
a) HCOOH
b) CH3COOH
c) C6H5COOH prepared commercially? Mention two uses of each.
7. Carry out the following conversions:
8. Explain the mechanism of the following reaction:a) Ethanol to ethanal.
b) Methanol to methanal.
c) Propan-2-ol to propanone.
d) Methanal to
i) ethanol
ii) benzyl alcohol.e) Ethanal to
i) propan-2-ol
ii) acetone.f) Acetone to
i) tert butyl alcohol
ii) 2-Methyl prop-1-ene.g) Benzaldehyde to
i) a- hydroxy phenyl acetic acid
ii) 3-phenyl propan-1-ol
iii) 1- phenyl ethanol.
iv) benzyl alcohol and sodium benzoate.
v) cinnamaldehyde.
vi) 1,3-Diphenyl prop- 2-en-1-one
vii) benzo phenone
viii) m-nitro benzaldehyde
ix) benzal acetophenone.
h) Butan-1-ol to butanal.
i) Cyclo hex-2-en-1-ol to cyclo hex-2-en 1-one.
j) Pentan-3-en-2-ol to pent-3-en-2-one.
k) But-2-ene to ethanal
l) Para nitro toluene to para nitro benzaldehyde.
m) Ethanal to butan-2-one.
n) Ethanal to butane- 1,3- diol.
o) Ethanal to but-2-enal
p) Ethanal to but-2-enoic acid.
q) Ethanal to butan-1-ol
r) Ethanal to butanoic acid.
s) Propanone to propene.
t) Propanal to butanone.
u) Ethanal to 2-hydroxy butanal.
v) Benzaldehyde to benzo phenone.
w) Benzoic acid to benzaldehyde.
x) Propanoic acid to propenoic acid.
y) Benzene to m-nitro aceto phenone.
z) Bromo benzene to 1-phenyl ethanol.
aa) Benzoic acid to m- nitro benzoic acid.
bb) Benzoic acid to benzyl amine.
cc) Para nitro benzoic acid to para nitro aniline.
dd) Hexanoic acid to hexane nitrle.
ee) Hexanoic acid to 1-amino pentane.
ff) Hept-1-ene to heptanal
gg) Hept-1-ene to hexanal.
hh) Hept-1-ene to heptanoic acid.
ii) Hept-1-ene to hexanoic acid.
jj) Ethene to ethanal.
kk) Propene to acetone.
ll) 2, 3- dimethyl but-2-ene to acetone.
mm) Ethanal to lactic acid.
nn) Ethanal to ethane.
oo) Ethanal to Ethanoic acid.
pp) Acetone to 4-hydroxy-4-methyl pentan-2-one.
qq) Benzaldehyde to benzyl alcohol and sodium benzoate.
rr) Methanal to methanol and sodium methanoate.
ss) Toluene to benzaldehyde.
tt) Ethyl benzene to benzoic acid.
uu) But-2-ene to ethanoic acid.
v v) Ethane nitrle to ethanoic acid.
ww) Methyl magnesium bromide to ethanoic acid.
xx) Para methyl aceto phenone to benzene-1,4- dicarboxylic acid.
yy) Cyclo hexene to hexane-1,6-dicarboxylic acid.
zz) Ethanoic acid to ethanol.
aaa) Propanoic acid to a- chloro propanoic acid.
bbb) Methanol to Ethanoic acid.
ccc) Benzoic acid to m-nitro benzoic acid.
ddd) Benzoic acid to m-bromo benzoic acid.
eee) Acetyl chloride to acetaldehyde.
fff) Benzoyl chloride to benzaldehyde.
mmm) Benzene to aceto phenone.
nnn) Benzene to benzo phenone.
ooo) Ethanoic acid to ethanoyl chloride.
sss) Ethanoic acid to ethanoic anhydride.
ttt) Ethanoic acid to ethyl ethanoate.
a) Ethanoic acid to ethanamine.
b) Hexane nitrle to 1-amino pentane.
c) Benzoic acid to benzo phenone.
d) Benzoic acid to
i) aceto phenone.
ii) benzaldehyde
iii) benzophenonee) Benzene to methyl benzoate.
f) Benzene to m- nitro benzoic acid.
g) Benzene to p- nitro benzoic acid.
h) Benzene to p- nitro benzaldehyde.
i) Benzene to phenyl acetic acid.
j) Ethanoyl chloride to propanone.
k) Benzene to benzaldehyde
l) Ethane nitrile to
i) ethanal
ii) propanalm) vinyl cyanide to prop2-enal
n) p-fluoro toluene to p-fluoro benzaldehyde.
o) cyclo hexanol to cycloheanone.
p) Hexan-1-ol to hexanal
q) Butan-1-ol to butanoic acid.
r) Benzyl alcohol to phenyl ethanoic acid.
s) 3-nitro bromo benzene to 3-nitro benzoic acid
t) 4- methyl acetophenone to benzene-1,4-dicarboxylic acid.
u) Butanal to butanoic acid.
v) Ethyl butanoate to ethanoic acid.
w) cyclo hexane to Hexane-1,6-dicarboxylic acid.
x) Benzene-1,4-dicarboxylic acid to pthalimide
CH3 CHO + HCN → CH3 CH CN
│
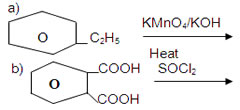
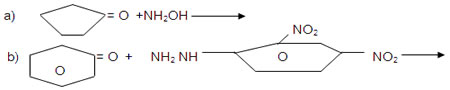
OH9. An organic compound A(C8H8O) gives orange red precipitate with 2,4-DNP reagent. It responds to iodoform test. It does not respond to Tollen’s reagent test. It does not decolorise bromine water. A on oxidation using CrO3 give B. Identify the compounds and write thw equations of the reactions involved.
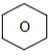
10. An organic compound C9H10O forms 2,4-DNP derivative, reduces Tollen’s reagent and Undergoes cannizaro reaction. On vigorous oxidation it gives Benzene-1,2-dicarboxylic acid. Identify the compound.
11. An organic compound contain 69.77% C and 11.63% H and the remaining O. Molecular mass of the compound is 86 u. It does not reduce Tollen’s reagent, gives positive iodoform test and respond to sodium bisulphate test. On oxidation it gives acetic acid and propanoic acid. Give the structure of the organic compound.
12. Arrange the following in the increasing order of property mentioned:
a) Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone( reactivity with HCN)
b) 2-Bromo butanoic acid, 3-Bromo butanoic acid, Butanoic acid, 3-Methyl propanoic acid (acid strength)
c) Benzoic acid, 4-Nitro benzoic acid, 3,4-Dinitro benzoic acid, 4-Methoxy benzoic acid (acid strength)
d) Ethanal, Propanal, Propanone, Butanone( increasing order of reactivity towards nucleophile)
e) Benzaldehyde, p-Tolualdehyde, p-Nitro benzaldehyde, Acetophenone(increasing order of reactivity towards nucleophile)
f) Butanal, Butanol, Ethoxy ethane and Butane (increasing order of boilimg point)
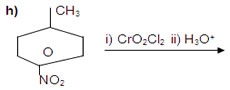
13. Complete each synthesis by giving missing starting material, reactant or product.
c) C6H5CHO + NH2CONHNH2
d) C6H5CHO + CH3CH2CHO
Dil NaOH
e) CH3COCH2COOC2H5
i) NaBH4 ii) H+f)
C6H5COC6H5
ii) Zn / H2O
14. Which acid of each pair would you expect to be stronger?
a) Ethanoicacid and fluoro ethanoic acid
b) Fluoro ethanoic acid and chloro ethanoic acid
c) 4- Fluoro butanoic acid and 3-Fluoro butanoic acid
d) p-Trifluoro benzoic acid and p- Methyl benzoic acid.
15. Arrange the following in the increasing order of boiling point: Ethanal, Ethanol, Ethoxy ethane and Propane
16. Predict the product formed when cyclohexanecarbaldehyde reacts with
a) PhMgBr+H3O+
b) Tollens’reagent
c) Semicarbazide and weak acid
d) excess ethanol and acid.
e) Zinc amalgam and dilute HCl
17. Give the products of the following reactions:
c) RCH=CHO + NH2CONHNH2
H+d) C6H5COCH3 + C2H55NH2
H+e) C6H6+C2H5COCl
Anhydr. AlCl3 / CS2f) (C6H5CH2)2Cd + 2 CH3COCl
g) CH3- C=CH
Hg2+,H2SO4
18. Identify the compounds A,B,C and D in the following reactions:
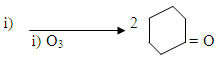
CH=C6H5
i) O3 ii) Zn / H2O NaOH/aq ethanol
A+B
C+ H2O
i) O3 ii) Zn / H2O
CD+A
Submitted By : Mr. R. Srinivas Vasudevamurthy
Email: [email protected]