CBSE Guess > Papers > Question Papers > Class X > 2009 > Science > Science
Q. 15. (a) What are ionic compounds? List any four physical properties of these compounds.
(b) What reaction happens when manganese dioxide is heated with aluminium powder? In which physical state is the metal produced in this reaction and why? 5
Ans. (a) The chemical bond formed by the transfer of electrons from one atom to another is known as an ionic bond and the compounds formed by such bonds are called ionic compounds.
Physical properties of ionic compounds:
1. Ionic compounds are usually crystalline solids because their oppositely charged ions attract one another strongly and form a regular crystal structure.
2. Ionic compounds have high melting points and high boiling points.
3. Ionic compounds are highly soluble in water.
4. Ionic compounds conduct electricity when dissolved in water or when melted because they contain charged particles i.e. ions.
(b) When manganese dioxide is heated with aluminium powder, manganese metal is produced
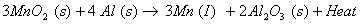
The reduction of manganese dioxide with aluminium is a highly exothermic reaction. A lot of heat is evolved therefore the manganese metal produced is in molten state.
Or
(a) What is an alloy? How does it differ from an amalgam?
(b) Name the method most widely used for refining impure metals.
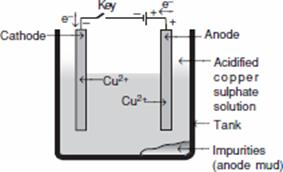
(c) Draw a labelled diagram to show the refining of copper. 5
Ans. (a) An alloy is a homogeneous mixture of two or more metals (or a metal and small amounts of non-metals). The properties of an alloy are different from the properties of the constituent metals.
An alloy of mercury metal with one or more other metals is known as an amalgam. e.g., a solution of sodium metal in liquid mercury metal is called sodium amalgam.
(b) The most widely used method for refining impure metals is
electrolytic refining.
(c) Labelled diagram to show the refining of copper
| Science 2009 Question Papers Class X | |||||||||
| Delhi | Outside Delhi | Compartment Delhi | Outside Delhi | Foreign | |||||
| Set 1 (PDF) | Set 1 (PDF) | Set 1 | Set 1 | Set 1 (PDF) | |||||
| Set 2 | Set 2 | ||||||||
| Set 3 | Set 3 | ||||||||