CBSE Guess > Papers > Question Papers > Class X > 2009 > Science > Science
Q. 13. (a) What are salts among chemical substances? Give an example of a salt derived from a strong acid and a weak base. State the behaviour of aqueous solution of this salt towards litmus solution.
(b) Why does hydrogen chloride gas not show acidic behaviour? 3
Ans. (a) A salt is a compound formed by the reaction between an acid and a base. Ammonium chloride (NH4Cl) is a salt of a strong acid, hydrochloric.acid (HCl), and a weak base, ammonium hydroxide (NH4OH). This salt is acidic in nature therefore it turns blue litmus red.
(b) The acidic behaviour of acids is due to the presence of hydrogen ions, H+ (aq) ions in them. The acids produce hydrogen ions only in the presence of water. So, in the absence of water, a substance will not form hydrogen ions and hence will not show its acidic behaviour. So HCl gas does not show acidic behaviour.
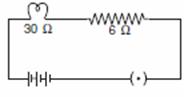
Q. 14. Draw a circuit diagram to show an electric lamp of resistance 30  and
a resistor of 6
and
a resistor of 6  connected in series with a battery of 6 V. Calculate
connected in series with a battery of 6 V. Calculate
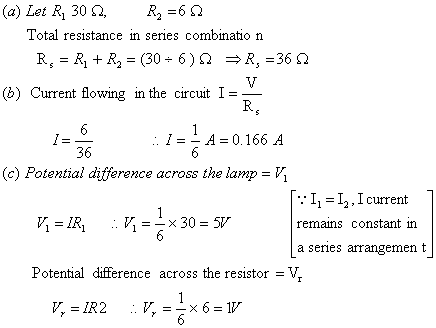
(a) total resistance in the circuit
(b) current flowing through the circuit
(c) potential difference separately across the lamp and the resistor. 3
Sol.
| Science 2009 Question Papers Class X | |||||||||
| Delhi | Outside Delhi | Compartment Delhi | Outside Delhi | Foreign | |||||
| Set 1 (PDF) | Set 1 (PDF) | Set 1 | Set 1 | Set 1 (PDF) | |||||
| Set 2 | Set 2 | ||||||||
| Set 3 | Set 3 | ||||||||