CBSE Guess > Papers > Question Papers > Class X > 2009 > Science > Science
Q.11. Name the products formed in each case when.
(a) hydrochloric acid reacts with caustic soda.
(b) granulated zinc reacts with caustic soda.
(c) carbon dioxide is passed into lime water. 3
Ans. (a) Sodium Chloride and Water.
(b) Sodium Zincate (Na2ZnO2) and H2 gas,
(c) Calcium Carbonate and Water.
Q. 12. What is ethanol? Draw the structure of ethanol molecule. How does
ethanol behave with the following :
(a) Sodium
(b) Excess of conc. sulphuric acid at 443 K
Write chemical equation for each reaction. 3
Ans.
- Ethanol is the second member of the homologous series of alcohol. It’s formula is C2H5OH. It is the most widely used alcohol. It’s common name is ethyl alcohol.
- Structure formula
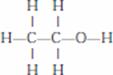
- Ethanol reacts with sodium to form sodium ethoxide and hydrogen gas

- When ethanol is heated with excess of conc. sulphuric acid at 443 K, it gets dehydrated to form ethene
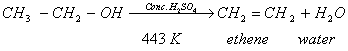
Q.13. A student placed a candle flame in front of a concave mirror at various distances from it and obtained the image of the candle flame on a white screen. He noted down his sets of observations in the following table:
| Distance of the flame from the mirror (cm) |
Distance of the screen from the mirror (cm) |
| 60 | 15 |
| 36 | 18 |
| 24 | 24 |
| 20 | 30 |
| 18 | 36 |
| 10 | 90 |
| Science 2009 Question Papers Class X | |||||||||
| Delhi | Outside Delhi | Compartment Delhi | Outside Delhi | Foreign | |||||
| Set 1 (PDF) | Set 1 (PDF) | Set 1 | Set 1 | Set 1 (PDF) | |||||
| Set 2 | Set 2 | ||||||||
| Set 3 | Set 3 | ||||||||