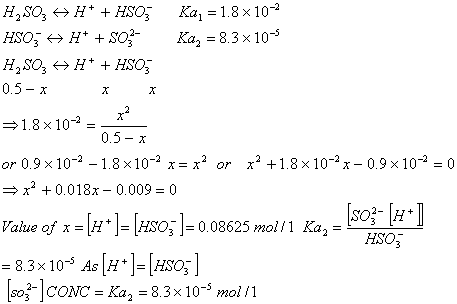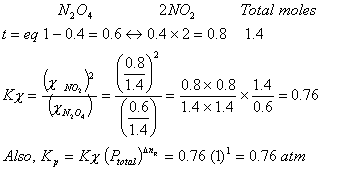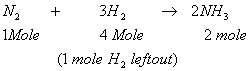All India Pre-Medical / Pre-Dental Entrance Examination (AIPMT) Papers
AIPMT Examination
AIPMT Solved / Unsolved Papers
AIPMT Important Links
AIPMT 2007 Chemistry Mains
Q. 1.
- Two silver rods are dipped in 1M HCl and 1M HNO3 . In which of the two acids will the silver rods dissolve under standard conditions? Given:
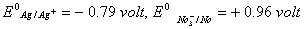
- A 0.1M acetic acid solution ionizes to 1.2%. What is its Ka ?
Sol. Silver rod will dissolve more readily in HNO3 than HCl because HCl is a non-oxidizing acid. Moreso if we establish ![]() for the reaction
for the reaction
![]()
As the![]() is positive the reaction would take place spontaneously under standard conditions
is positive the reaction would take place spontaneously under standard conditions
Q. 2.
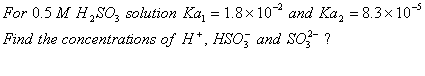
-
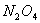 dissociates with a degree of dissociation as 0.4. Establish
dissociates with a degree of dissociation as 0.4. Establish 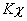 . Relation between
. Relation between 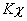 and Kp and the value of Kp . Given total pressure = 1 atm and T = 315 K
and Kp and the value of Kp . Given total pressure = 1 atm and T = 315 K - 1 Mole of nitrogen and 4 mole of Hydrogen react to form ammonia in a 20 ltr vessel. 10 litres of water are added and the vessel properly shaken. What will be the pressure of the residual gases?
Sol.
The formed NH3 shall dissolve in water on shaking
So, ![]()
When 10 litre H2O is added, the effective volume of the vessel occupied by H2
gas will be 20 – 10 = 10 ltr
Q. 3.
- An electron in which orbit of lithium will have same energy as an electron in the second orbit of hydrogen?
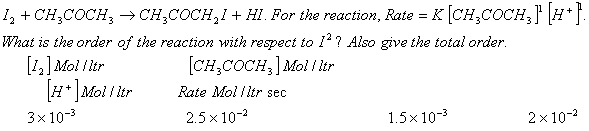 Also find the value and unit of the rate constant from the data given above.
Also find the value and unit of the rate constant from the data given above.- For a photoelectron, the frequency is given by the expression
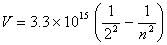 . If the wavelength of
. If the wavelength of
the photoelectron is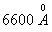 , what will be the value of ‘n’?
, what will be the value of ‘n’?
Sol.
- Energy of electron in second orbit of hydrogen
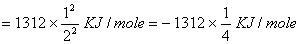
For lithium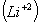 ion energy should be
ion energy should be 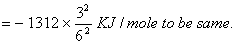
The electron must be in the sixth Bohr orbit of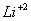 .
. - As
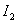 does not feature in the rate law expression so, the order of the reaction w.r.t
does not feature in the rate law expression so, the order of the reaction w.r.t 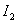 will be zero.The total order of the reaction = 2
Rate
will be zero.The total order of the reaction = 2
Rate
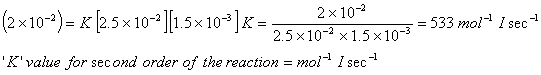
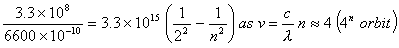
Q. 4.
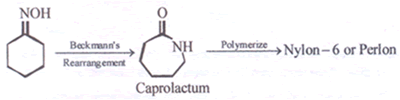
- Complete the reaction given below
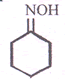
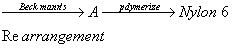
Cyclohexanonoxime - Identify which of the following given compounds is optically active
(i)2-chloro 3-methyl pent -1, 4-diene
(ii) 3-methyl 3-hydroxy pentanol
(iii) 2-chloro 2-methyl butane - Convert:
(i)
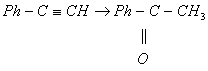
(ii)
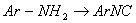
- An alkene
 reacts withHBr both in the presence and absence of peroxides to give the same product. Identify the alkene.
reacts withHBr both in the presence and absence of peroxides to give the same product. Identify the alkene.
Sol.
- (i)
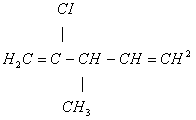
(Optically active)
(ii)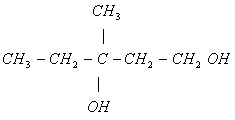
(Optically active)
(iii)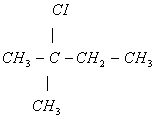
(Optically inactive) - (i)
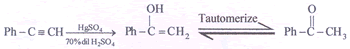
(ii)
- As 2-butene
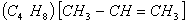 is symmetric so, its reaction with HBr with or without a peroxide would yield the same product 2-Bromo butane.
is symmetric so, its reaction with HBr with or without a peroxide would yield the same product 2-Bromo butane.
Q. 5.
- (i)
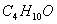 is produced on reaction of an alkane with
is produced on reaction of an alkane with 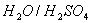 , which is not resolvable into optical isomers. Identify the compound?
, which is not resolvable into optical isomers. Identify the compound? - (ii) Make two possible dipeptides from the amino acids given below:
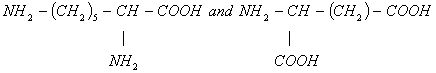
- The amino acid alanine when kept in a solution with pH less than its isoelectric point it coagulates at the cathode and if pH is greater than isoelectric point it coagulates at anode. Explain this phenomenon.
- Which out of 1-Butene and 2-Butene react easily with
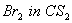 and why?
and why?
Sol.
- The Alkanes must be
- The two dipeptides would be
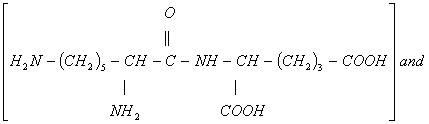
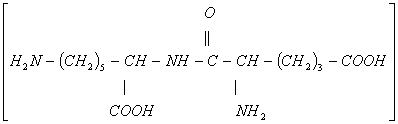
- (iii) In an acidic solution alanine exists as
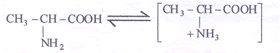
As the colloid is now positively charged it coagulates at the negative electrode (cathode) when (Iso electric pt)
In basic media
(Iso electric pt)
In basic media 
This colloid is now negatively charged, so it coagulates at the anode (positive electrode) - 1-butene is less crowded than 2-butene so will react more readly with
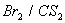
Q. 6.
- Why 1-Butyne gives sodium salt with
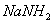 but 2-butyne does not?
but 2-butyne does not? - Draw the structures for DNA purines?
Sol.
- The terminal hydrogen in 1-Butyne is acidic. There is no such available hydrogen is 2-Butyne.

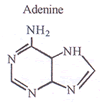
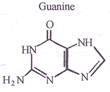
Q. 7.
- Why
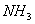 is more soluble in water than
is more soluble in water than 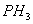 ?
? - Why
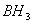 dimerizes but
dimerizes but 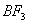 does not?
does not? - The complex
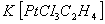 has 3 chlorine atoms bonded to platinum. Why is the chlorine atom lying opposite to ethene have higher bond length?
has 3 chlorine atoms bonded to platinum. Why is the chlorine atom lying opposite to ethene have higher bond length?
Sol.
- Nitrogen in
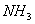 is electron dense and electronegative, it has the ability to hydrogen bond with water. Phosphorous does not show hydrogen bonding.
is electron dense and electronegative, it has the ability to hydrogen bond with water. Phosphorous does not show hydrogen bonding. -
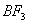 is stabilized by
is stabilized by 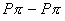 back bonding due to which the electron deficiency of the central boron is satisfied. Backbonding is not seen in BH3 due to which it involves in bridge bonding.
back bonding due to which the electron deficiency of the central boron is satisfied. Backbonding is not seen in BH3 due to which it involves in bridge bonding. - Ethene is
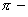 a donor ligand when the
a donor ligand when the 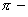 cloud of ethane is polarized for donation into the d-orbitals of Pt, the chlorine lying opposite to it experiences repulsions. This causes the bond length to increase.
cloud of ethane is polarized for donation into the d-orbitals of Pt, the chlorine lying opposite to it experiences repulsions. This causes the bond length to increase.
Q. 8.
- Why
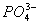 ions exist but
ions exist but 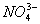 ions don’t?
ions don’t? - Why
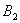 is paramagnetic but
is paramagnetic but 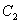 is not?
is not? - For a octahedral fields splitting
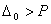 when the pairing energy is less and
when the pairing energy is less and 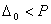 when pairing energy is higher. Explain the spin magnetic moments acquired by
when pairing energy is higher. Explain the spin magnetic moments acquired by 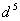 and
and 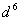 configurations of metal ions in this field.
configurations of metal ions in this field.
Sol.
- In order to form
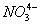 , nitrogen will have to exceed its octet and utilize d-orbitals to form multiple bonds with oxygen. As nitrogen does not have d-orbitals this is not possible.
, nitrogen will have to exceed its octet and utilize d-orbitals to form multiple bonds with oxygen. As nitrogen does not have d-orbitals this is not possible. 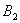 has a total of
has a total of 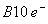 S with a M.O configuration
S with a M.O configuration
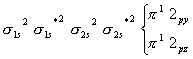
 has 12 electrons due to which the
has 12 electrons due to which the  bonding molecular orbitals fill up completely making it diamagnetic.
bonding molecular orbitals fill up completely making it diamagnetic.- In a strong field
 Complex will be paramagnetic.
Complex will be paramagnetic.
Spin magnetic moment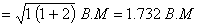
 Complex will be diamagnetic.
Complex will be diamagnetic.
Spin magnetic moment = Zero In a weak field
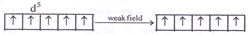
Spin magnetic moment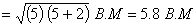

Spin magnetic moment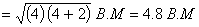
Q. 9.
- The empirical formula of an insoluble compound is
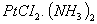 . On churning this compound with
. On churning this compound with 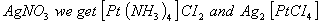 . The molecular formula of the compound will be?
. The molecular formula of the compound will be? - Out of trimethyl amine and triethyl phosphine, which one has higher dipole moment?
Sol.
- From the products we understand that the complex must be a higher empirical derivative of
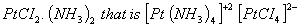
The complex is tetraammineplatinum (II) tetrachloroplatinate (II) 
Both molecules shown above are pyramidal. Due to repulsions that arise between three Ethyl groups in triethyl phosphine the bond angle is larger which results in a lower Dipole moment.
Q. 10.
- Why is
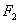 more reactive than
more reactive than 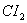 ?
? - Why is
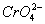 more oxidizing than
more oxidizing than 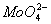
- Out of
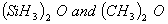 which is more basic and why?
which is more basic and why?
Sol.
- Due to high charge density of the Fluorine atom, inner electrons repel each other in the F – F bond making it weak and increasing the reactivity of
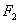 .
. - Most oxo ions of chromium are very strongly oxidizing. The most stable oxidation state of chromium is (+III), this results in spontaneous reduction of
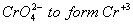 . For molybdenum (+III) state is uncommon, in fact 2-
. For molybdenum (+III) state is uncommon, in fact 2- 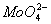
has Mo in (+VI) stat e which is the most stable. 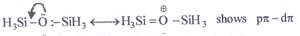
bonding due to the presence of vacant d-orbitals with Si. This however is not possible with carbon in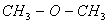 due to absence of d-orbitals making it more basic.
due to absence of d-orbitals making it more basic.