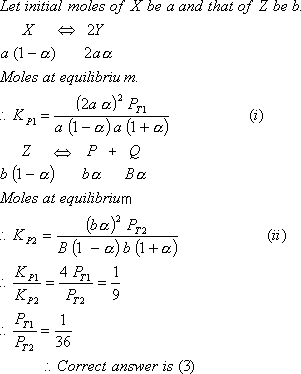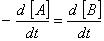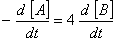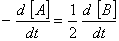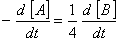All India Engineering / Architecture Entrance Examination (AIEEE)
CBSE Guess > AIEEE > AIEEE Papers > 2008 > Chemistry
AIEEE 2008 Chemistry
Q. 19. At 800C, the vapour pressure of pure liquid 'A' is 520 mm Hg and that of pure liquid 'B' is 1000 mm Hg. If a mixture solution of 'A' and 'B' boils at 800C and 1 atm pressure, the amount of 'A' in the mixture is (1 atm = 760 mm Hg)
- 48 mol percent
- 50 mol percent
- 52 mol percent
- 34 mol percent
Sol:
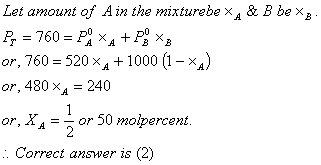
Q. 20. For a reaction 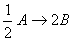 , rate of disappearance of 'A' is related to the rate of appearance of 'B' by the expression
, rate of disappearance of 'A' is related to the rate of appearance of 'B' by the expression
Sol:
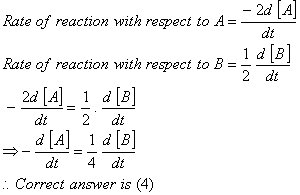
Q. 21. The equilibrium constants 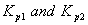 for the reactions
for the reactions 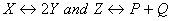 respectively are in the ratio of 1 : 9. If the degree of dissociation of X and Z be equal then the ratio of total pressures at these equilibria is
respectively are in the ratio of 1 : 9. If the degree of dissociation of X and Z be equal then the ratio of total pressures at these equilibria is
- 1 : 3
- 1 : 9
- 1 : 36
- 1 : 1
Sol: