AIEEE 2008 Chemistry
Q. 22. In context with the industrial preparation of hydrogen from water gas (CO + H2), which of the following is the correct statement?
Sol:
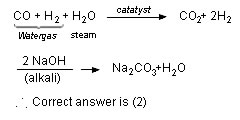
Q. 23. In which of the following octahedral complexes of Co (atomic number 27), will the magnitude of 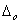 be the highest?
be the highest?
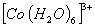
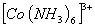
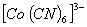
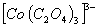
Sol: Magnitude of 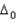 will be heighest with the strongest ligand. In the given context CN- is the strongest ligand and would lead to a greater seperatin between t2g and cg orbitals.
will be heighest with the strongest ligand. In the given context CN- is the strongest ligand and would lead to a greater seperatin between t2g and cg orbitals.
Q. 24. The coordination number and the oxidation state of the element 'E' in the complex 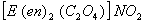 (where (en) is ethylene diamine) are, respectively,
(where (en) is ethylene diamine) are, respectively,
Sol: Ethyldiamine and oxalateion are both bidentate legends. Co-ordination number of E in the complex.
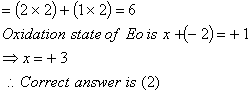
Q. 25. Identify the wrong statement in the following:
Sol: Ozone layer does not permit the ultraviolet radiation from the sum to the earth.