AIEEE 2010 Chemistry
Q. 5. If sodium sulphate is considered to be completely dissociated into cations and anions in aqueous solution, the change in freezing point of water (kf = 1.86 K kgmol-1), when 0.01 mol of sodium sulphate is dissolved in 1 kg of water, is (Kf = 1.86 K kg mol-1)
Sol :
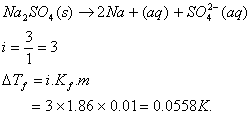
Answer: (2)
Q. 6 . In the chemical reactions,

The compounds ‘A’ and ‘B’ respectively are
Sol :

Answer: (3)