AIEEE 2010 Chemistry
Q. 7. The energy required to break one mole of Cl–Cl bonds in Cl3 is 242 kJ mol-1. The longest wavelength of light capable of breaking a single Cl–Cl bond is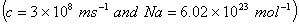
Sol :
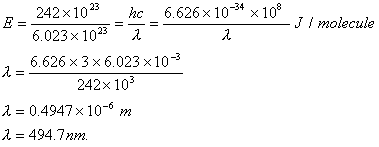
Answer: (4)
Q. 8. Which one of the following has an optical isomer?
Sol :
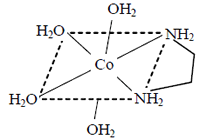
Square planar complexes of Zn2+ cannot show optical isomerism. So, options (1) and (4) are ruled out.
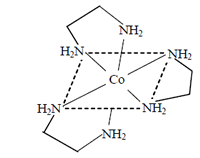
will have two planes of symmetry, so it is optically inactive. Thus optically active compound is
as it has no plane of symmetry.
Answer: (2)