AIEEE 2010 Chemistry
Q. 11. Solubility product of silver bromide is 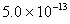 . The quantity of potassium bromide (molar mass taken as 120 g mol-1) to be added to 1 litre of 0.05 M solution of silver nitrate to start the precipitation of AgBr is.
. The quantity of potassium bromide (molar mass taken as 120 g mol-1) to be added to 1 litre of 0.05 M solution of silver nitrate to start the precipitation of AgBr is.
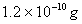
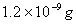
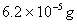
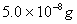
Sol :
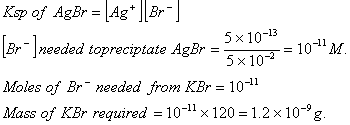
Answer: (2)
Q. 12. At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10-11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2from a solution of 0.001 M Mg2+ ions?
Sol :
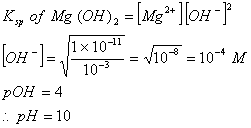
Answer: (2)