AIEEE 2010 Chemistry
Q. 13. Out of the following, the alkene that exhibits optical isomerism is
Sol.: Only 3-methyl-1-pentene has a chiral carbon atom, responsible for its optical rotation.
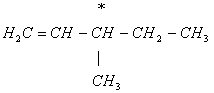
Answer: (3)
Q. 14. The correct order of 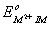 values with negative sign for the four successive elements Cr, Mn, Fe and Co is.
values with negative sign for the four successive elements Cr, Mn, Fe and Co is.
Sol :
The value of 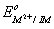 for given metal ions are}
for given metal ions are}
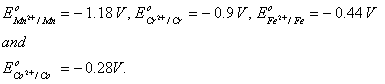
The correct order of 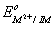 values without considering negative sign would be
values without considering negative sign would be 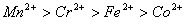 .
.
Answer: (1)