All India Engineering / Architecture Entrance Examination (AIEEE)
CBSE Guess > AIEEE > AIEEE Papers > 2007 > Chemistry
AIEEE 2007 Chemistry
Q. 12. Presence of a nitro group in a benzene ring
- activates the ring towards electrophilic substitution.
- renders the ring basic.
- deactivates the ring towards nucleophilic substitution.
- deactivates the ring towards electrophilic substitution.
Sol: −NO2 group in benzene ring shows −I and −R effect, which deactivates the ring towards electrophilic substitution but activates it towards nucleophilic substitution. Correct choice: (4)
Q. 13. In which of the following ionization processes, the bond order has increased and the magnetic behaviour has changed?
Sol:
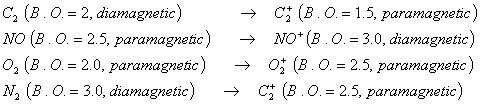
Correct choice: (2)
Q. 14. The actinoids exhibit more number of oxidation states in general than the lanthanoids. This is because
- the 5f orbitals are more buried than the 4f orbitals.
- there is a similarity between 4f and 5f orbitals in their angular part of the wave function.
- the actinoids are more reactive than the lanthanoids.
- the 5f orbitals extend further from the nucleus than the 4f orbitals.
Sol: The actinoids exhibit more number of oxidation states than lanthanoids because they can loose more number of electrons as the 5f orbital electrons are held less strongly than the 4f orbital electrons. Correct choice: (4)
Q. 15. Equal masses of methane and oxygen are mixed in an empty container at ![]() . The fraction of the total pressure exerted by oxygen is
. The fraction of the total pressure exerted by oxygen is
Sol: Let the mass of methane and oxygen be x g each.

Correct choice: (3)