All India Engineering / Architecture Entrance Examination (AIEEE)
CBSE Guess > AIEEE > AIEEE Papers > 2007 > Chemistry
AIEEE 2007 Chemistry
Q. 16. . A 5.25% solution of a substance is isotonic with a 1.5% solution of urea (molar mass = 60 g mol–1) in the same solvent. If the densities of both the solutions are assumed to be equal to 1.0 g cm–3, molar mass of the substance will be
- 90.0 g mol–1
- 115.0 g mol–1
- 105.0 g mol–1
- 210.0 g mol–1
Sol: Since the two solutions are isotonic, their concentrations will be same. ![]() Correct choice: (4)
Correct choice: (4)
Q. 17. Assuming that water vapour is an ideal gas, the internal energy change ![]() when 1 mol of water is vapourised at 1 bar pressure and 1000C,(Given: Molar enthalpy of vapourisation of water at 1 bar and 373 K = 41 kJ mol−1 and R = 8.3 J mol−1K−1) will be:
when 1 mol of water is vapourised at 1 bar pressure and 1000C,(Given: Molar enthalpy of vapourisation of water at 1 bar and 373 K = 41 kJ mol−1 and R = 8.3 J mol−1K−1) will be:
- 4.100 kJ mol−1
- 3.7904 kJ mol−1
- 37.904 kJ mol−1
- 41.00 kJ mol−1
Sol:
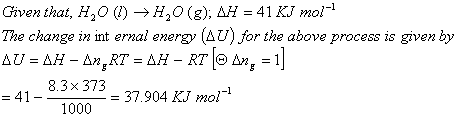
Correct choice: (3)
Q. 18. In a saturated solution of the sparingly soluble strong electrolyte AgIO3(Molecular mass = 283) the equilibrium which sets in is ![]() If the solubility product constant K spof AgIO3 at a given temperature is, what is the mass of AgIO3contained in 100 ml of its saturated solution?
If the solubility product constant K spof AgIO3 at a given temperature is, what is the mass of AgIO3contained in 100 ml of its saturated solution?
- 28.3 × 10–2 g
- 2.83 × 10–3 g
- 1.0 × 10–7 g
- 1.0 × 10–4 g
Sol:
![]()
If ‘s’ is the solubility of AglO3 in a saturated solution, then
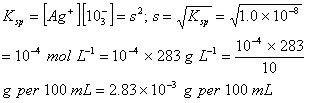
Correct choice: (2)