AIPMT 2010 Solved Papers
AIPMT Examination
AIPMT Solved / Unsolved Papers
AIPMT Important Links
AIPMT 2010 Chemistry
Q. 16. A solution of sucrose (molar mass = 342 g mol−1) has been prepared by dissolving 68.5 g of sucrose in 1000 g of water. The freezing point of the solution obtained will be: (Kf for water = 1.86 K kg mol−1)
- −0.372oC
- −0.520oC
- + 0.372oC
- −0.570oC
Sol.
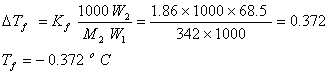
Answer: (1)
Q. 17. An increase in equivalent conductance of a strong electrolyte with dilution is mainly due to:
- increase in ionic mobility of ions
- 100% ionisation of electrolyte at normal dilution
- increase in both i.e. number of ions and ionic mobility of ions
- increase in number of ions.
Answer : (1)
Q. 18. Oxidation states of P in H4P2O5, H4P2O6, H4P2O7 are respectively:
- +3, +5, +4
- +5, +3, + 4
- +5, +4, +3
- +3, +4, +5
Sol.
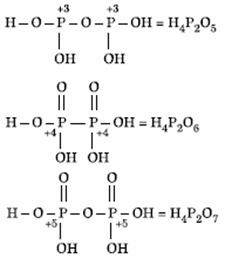
Answer : (4)