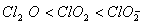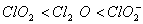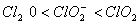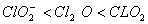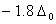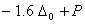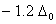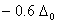AIPMT Examination
AIPMT Solved / Unsolved Papers
AIPMT Important Links
AIPMT 2010 Chemistry
Q. 19. The correct order of increasing bond angles in the following species are:
Sol:
The correct order of increasing bond angle is 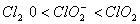
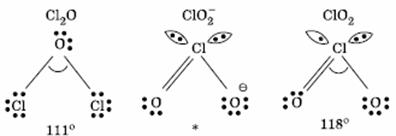
* In 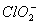 there are 2 lone pairs of electrons present on the central chlorine atom. Therefore the bond angle in
there are 2 lone pairs of electrons present on the central chlorine atom. Therefore the bond angle in 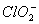 _ is less than 118° which is the bond angle in
_ is less than 118° which is the bond angle in 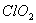 which has less number of electrons on chlorine.
which has less number of electrons on chlorine.
Answer : (3)
Q. 20. Which of the following alkaline earth metal sulphates has hydration enthalpy higher than the lattice enthalpy?
- CaSO4
- BeSO4
- BaSO4
- SrSO4
Sol:
Be+2 is very small, hence its hydration enthalpy is greater than its lattice Enthalpy
Correct choice : (2)
Q. 21. Crystal field stabilization energy for high spin d4 octahedral complex is:
Sol:
d4 in high spin octahedral complex
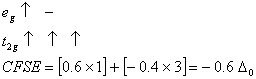
Answer : (4)