Question 20: Complete the following reactions:
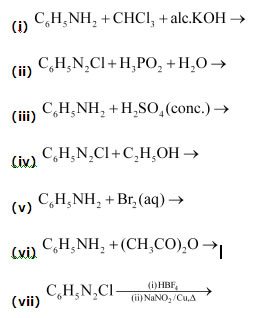
Answer :
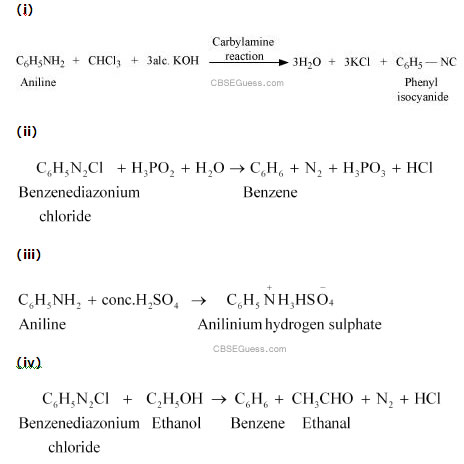
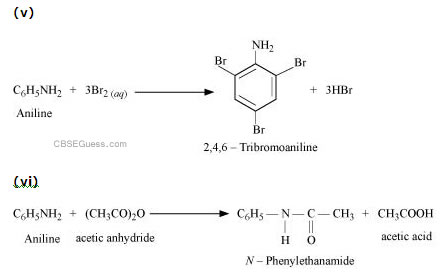
(vii)

Question 21: Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
Answer :
Gabriel phthalimide synthesis is used for the preparation of aliphatic primary amines. It involves nucleophilic substitution (SN2) of alkyl halides by the anion formed by the phthalimide.
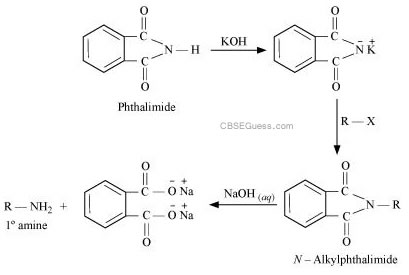
But aryl halides do not undergo nucleophilic substitution with the anion formed by the phthalimide.
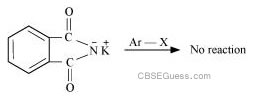
Hence, aromatic primary amines cannot be prepared by this process.
Question 22: Write the reactions of (i) aromatic and (ii) aliphatic primary amines with nitrous acid
Answer :
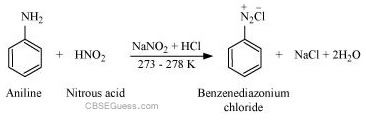
(i) Aromatic amines react with nitrous acid (prepared in situ from NaNO2 and a mineral acid such as HCl) at 273 − 278 K to form stable aromatic diazonium salts i.e., NaCl and H2O.
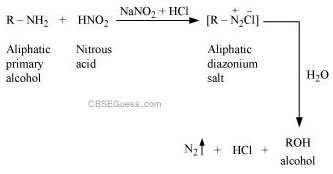
(ii) Aliphatic primary amines react with nitrous acid (prepared in situ from NaNO2 and a mineral acid such as HCl) to form unstable aliphatic diazonium salts, which further produce alcohol and HCl with the evolution of N2 gas.
Question 23: Give plausible explanation for each of the following:
(i) Why are amines less acidic than alcohols of comparable molecular masses?
(ii) Why do primary amines have higher boiling point than tertiary amines?
(iii) Why are aliphatic amines stronger bases than aromatic amines?
Answer :
(i) Amines undergo protonation to give amide ion.
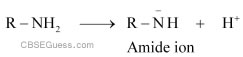
Similarly, alcohol loses a proton to give alkoxide ion.
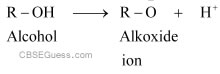
In an amide ion, the negative charge is on the N-atom whereas in alkoxide ion, the negative charge is on the O-atom. Since O is more electronegative than N, O can accommodate the negative charge more easily than N. As a result, the amide ion is less stable than the alkoxide ion. Hence, amines are less acidic than alcohols of comparable molecular masses.
(ii) In a molecule of tertiary amine, there are no H−atoms whereas in primary amines, two hydrogen atoms are present. Due to the presence of H−atoms, primary amines undergo extensive intermolecular H−bonding.
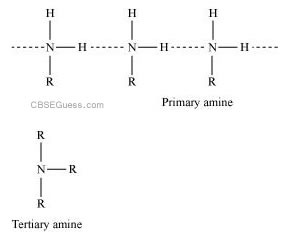
As a result, extra energy is required to separate the molecules of primary amines. Hence, primary amines have higher boiling points than tertiary amines.
(iii) Due to the −R effect of the benzene ring, the electrons on the N- atom are less available in case of aromatic amines. Therefore, the electrons on the N-atom in aromatic amines cannot be donated easily. This explains why aliphatic amines are stronger bases than aromatic amines.
Prepared By: Mr. MANISH TULI
mail to: [email protected]