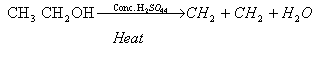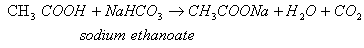CBSE Guess > Papers > Question Papers > Class X > 2008 >Science > Science Theory
(c) Soaps are ineffective in hard water because
(i) The formation of lather is necessary for removing dirt from clothes during the washing of clothes. Soap does not give lather with hard water as it reacts with the calcium and magnesium ions present in
hard water to form insoluble precipitates of calcium and magnesium salts of fatty acids.
(ii) The scum (or precipitate) formed by the action of hard water on soap sticks to the clothes being washed and it interferes with the cleaning ability of soap. This makes the cleaning of clothes difficult.
or
Complete the following equations
(i) 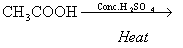
(ii) 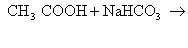
(iii) 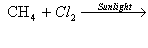
Write the name of the following :
(i) CH3CH2COOH
(ii) CH3CH2Br
(iii) Draw the electron dot structure of ethene (C2H4).
Ans.
(b)
- CH3CH2COOH Propanoic Acid
- CH3CH2Br Bromo ethane
| Science 2008 Question Papers Class X | |||||||
| Delhi | Outside Delhi | Compartment Delhi | Compartment Outside Delhi | ||||
| All Sets (PDF) | Set 1 | Set 1 | |||||
| Set 2 | Set 2 | ||||||
| Set 3 | Set 3 | ||||||