CBSE Guess > Papers > Question Papers > Class X > 2008 >Science > Science Theory
Q. 8. A compound ‘X’ of sodium is commonly used in kitchen for making crispy pakoras. It is also used for curing acidity in the stomach. Identify‘X’. What is its chemical formula? State the reaction which takes place when it is heated during cooking.
Ans. (i) The compound ‘X’ is sodium hydrogen-carbonate commonly known as
baking soda.
(ii) The chemical formula of baking soda is NaHCO3.
(iii) Action of heat : When sodium hydrogen-carbonate is heated during the
cooking of food, it decomposes to give sodium carbonate with the
evolution of carbon-dioxide gas.
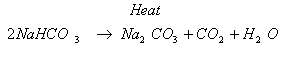 |
|||
| Sodium hydrogencarbonate (baking soda) |
carbonate Sodium | Carbon dioxide | Water |
Q. 9. An electron enters a uniform magnetic field at right angles to it as shown in the figure. In which direction will this electron move? State the principle applied by you in finding the direction of motion of the electron.

Ans. The electron will move in the outward direction.
The principle applied is Fleming’s Left-hand rule :
According to Fleming’s Left hand Rule : Hold the forefinger, the centre finger and the thumb of your left hand at right angles to one another. Adjust the hand in such a way that the forefinger points in the direction
of magnetic field and the centre finger points in the direction of current, then the direction in which the thumb points gives the direction of force acting on the conductor and this force causes motion in the conductor.
Q. 10. Give reason of the following :
(a) Gold and silver are used to make jewellery.
(b) Carbonate and sulphide ores are usually converted into oxides prior
to reduction during the process of extraction.
Ans. (a) Gold and silver are used to make jewellery because .
(i) Gold and silver are the most malleable metals.
(ii) Gold and silver are the most ductile metals.
(iii) Gold and silver do not react with air, water and dil. acids. Therefore
these metals do not corrode when exposed to atmosphere.
(iv) Gold does not tarnish and retains its luster for years.
(b) Carbonate and sulphide ores are generally converted into oxides prior
to reduction during the process of extraction because it is easier to
obtain metals from their oxides than from carbonates or sulphides.
| Science 2008 Question Papers Class X | |||||||
| Delhi | Outside Delhi | Compartment Delhi | Compartment Outside Delhi | ||||
| All Sets (PDF) | Set 1 | Set 1 | |||||
| Set 2 | Set 2 | ||||||
| Set 3 | Set 3 | ||||||