CBSE Guess > Papers > Question Papers > Class X > 2008 >Science > Science Theory
Ans. (a) Fleming’s Right hand Rule : See Q.11 (b) (S.A.T.), Chapter 13 [Page 147
(b) (i) Name the electric device that converts mechanical energy into electrical energy.
(ii) Write the principle involved in this device.
(c) An electric geyser
Power, P = 2kW =2 × 1000 W = 2000 W
Voltage, V = 220 V, Current, I = ?
P = V × I 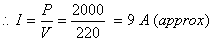
Now the current drawn by the geyser is 9 amperes (approx.) which is very high, but the fuse in this circuit is of only 5 A capacity. So, when a very high current of 9 A flows through the 5 A fuse, the fuse wire will get heated too much, will melt and break the circuit, cutting down the power supply.
Q.16. (a) Describe an activity to find out which metal is more reactive — iron or copper.
(b) Arrange the following metals in decreasing order of their reactivity :
Fe, Zn, Na, Cu, Ag
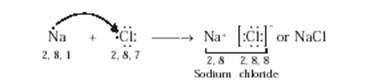
(c) Show the formation of NaCl from Na and Cl atoms by the transfer of electrons.
(d) Why do ionic compounds have high melting points?
Ans. (a) Activity
- Take about 10 mL of deep blue copper sulphate solution in a test tube.
- Take a big iron nail and clean its surface by rubbing with a sand paper.
- Put the cleaned iron nail in the test tube containing copper sulphate solution. Allow the iron nail to remain in the solution for about half an hour.
- After half an hour, the iron nail is taken out. It is observed that the iron nail is covered with a red-brown layer of copper metal.
- In the test tube the original deep blue colour of copper sulphate solution has faded and the solution turns light green due to the formation of iron sulphate.
- This occurs because iron displaces copper from copper sulphate solution. The deep blue colour of the solution fades due to the formation of light green solution of iron sulphate. A red-brown coating of copper metal is formed on the surface of iron nail. This displacement reaction shows that iron is more reactive than copper.
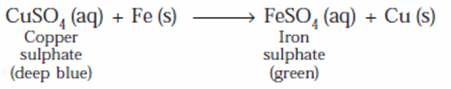
(b) Na > Zn > Fe > Cu > Ag
(c) The electronic configuration of chlorine is 2, 8, 7. Chlorine atom thus has 7 electrons in its outermost shell and needs 1 more electron to achieve the stable nearest inert gas configuration. So, a chlorine atom takes 1 electron (from the sodium atom) and forms a negatively charged chloride ion, Cl–
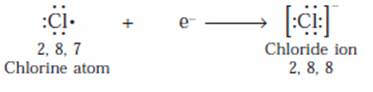
(d) Ionic compounds have high melting points because the ionic compounds are made up of positive and negative ions. There is a strong force of attraction between the oppositely charged ions, so a lot of heat energy is required to break this force of attraction and to melt or boil the ionic compound. Due to this, ionic compounds have high melting points and high boiling points.
| Science 2008 Question Papers Class X | |||||||
| Delhi | Outside Delhi | Compartment Delhi | Compartment Outside Delhi | ||||
| All Sets (PDF) | Set 1 | Set 1 | |||||
| Set 2 | Set 2 | ||||||
| Set 3 | Set 3 | ||||||