CBSE Guess > Papers > Question Papers > Class X > 2008 >Science > Science Theory
(a) Which atom is bigger, Na or Mg? Why?
(b) Identify the most (i) metallic and (ii) non-metallic element in Period .
Ans. (a) Na is bigger than Mg because on moving from left to right in a period, the atomic number of elements increases which means that the number of protons and electrons in the atom increases. (The extra electrons being added to the same shell.)
(b) (i) Most metallic element is Na, Most non-metallic element is Cl. Because on moving from left to right in a period the nuclear charge increases thus the valence electrons are pulled in more strongly by the nucleus and it becomes more and more difficult for the atoms to lose electrons so tendency of atoms to lose electrons (i.e. metallic character) decreases on moving from left to right in a period. On the other hand, due to increased nuclear charge, it becomes easier for the atoms to gain electrons. So the tendency to gain electrons (i.e. non-metallic character) increases on moving from left to right in a period.
Q. 10. The elements of the second period of the Periodic Table are given below :
Li Be B C N O F
(a) Give reason to explain why atomic radii decreases from Li to F.
(b) Identify the most (i) metallic and (ii) non-metallic element.
Ans. 2nd period → Li Be B C N O F
(a) Atomic radii decreases from Li to F because on moving from left to right, the atomic number of elements increases which means the number of protons and electrons in the atoms increases and extra electrons are being added to the same shell. Due to large positive charge on the nucleus, the electrons are pulled in more close to the nucleus and the size of the atom decreases.
(b) Most metallic element is Li, most non-metallic element is F. On moving from left to right in a periodic table the metallic character of elements decreases but the non-metallic character increases. Metals lose electrons and form positive ions so metals are electropositive whereas non-metals accept electrons and form negative ions, so nonmetals are electronegative.On moving from left to right in a period the nuclear charge (positive) increases as the number of protons increases, therefore the valence electrons are pulled in more strongly by the nucleus so the tendency of atoms to lose electrons decreases.
Q.11. (a) Name the compound CH3COOH and identify its functional group.
(b) Give a chemical test to identify this compound.
(c) Name the gas evolved when this compound acts on solid sodium carbonate. How would you identify this gas?
Ans. (a) CH3COOH = Ethanoic acid The functional group present is carboxylic acid (– COOH).
(b) When acetic acid reacts with ethyl alcohol in the presence of sulphuric acid to form ethyl acetate an ester which has a fruity smell, this fruity smell shows that the organic compound is a carboxylic acid.
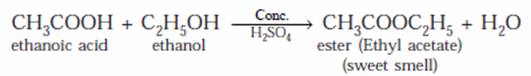
(c) Test to identify ethanoic acid. About 0.5 g of sodium carbonate is taken in a boiling tube and 2 mL of dilute ethanoic acid is added to it. It is observed that brisk effervescence of carbon dioxide gas is produced. If this gas is passed through lime water, the lime water turns milky (characteristic property of CO2). If a substance produces brisk effervescence of CO2 gas, then it will be an organic acid.
| Science 2008 Question Papers Class X | |||||||
| Delhi | Outside Delhi | Compartment Delhi | Compartment Outside Delhi | ||||
| All Sets (PDF) | Set 1 | Set 1 | |||||
| Set 2 | Set 2 | ||||||
| Set 3 | Set 3 | ||||||