CBSE Guess > Papers > Question Papers > Class X > 2008 >Science > Science Theory
Or
(a) Give an example of a metal which (i) can be easily cut with a knife. (ii) is a liquid at room temperature.
(b) Write chemical equation for the reaction when (i) steam acts on red hot iron. (ii) zinc is added to iron (II) sulphate solution.
(c) What are alloys?
(d) Why are food cans coated with tin and not zinc?
Ans. (a) (i) Sodium metal can be easily cut with a knife.
(ii) Mercury is a liquid at room temperature.
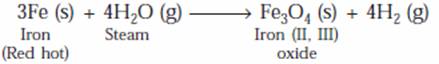
(b) (i) Red hot iron reacts with steam to form iron (II, III) oxide and hydrogen.
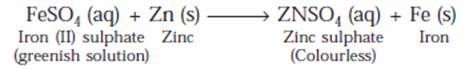
(ii) When Zinc is added to iron (II) sulphate solution, the greenish colour of iron (II) sulphate fades gradually due to the formation of colourless zinc sulphate solution and iron metal is deposited on zinc.
(c) The homogeneous mixture of two or more metals or metal and a nonmetal to improve the properties is called an alloy.
(d) Food cans are coated with tin and not zinc because zinc is a very reactive metal whereas tin is a less reactive metal. So the food contents like water and acids do not react with tin coating.
Section B
Q.17. What advantage over an aquatic organism does a terrestrial organism have with regard to obtaining oxygen for respiration?
Ans. The aquatic organisms use the oxygen dissolved in water through a special organ called gills.
Q.18. What is the minimum speed of wind to run a wind-mill to maintain the necessary speed of turbine in an electric generator?
Ans. The minimum speed of wind to run a wind-mill is 15 km/h to maintain the necessary speed of turbine in an electric generator.
Q.19. How is nuclear energy generated during nuclear fusion?
Ans. During fusion, two nuclei of light element combine to form a heavy nucleus with the release of tremendous amounts of energy. There is some loss of mass during fusion process which is transformed into tremendous amount of energy (i.e. 1 amu = 931 MeV).
| Science 2008 Question Papers Class X | |||||||
| Delhi | Outside Delhi | Compartment Delhi | Compartment Outside Delhi | ||||
| All Sets (PDF) | Set 1 | Set 1 | |||||
| Set 2 | Set 2 | ||||||
| Set 3 | Set 3 | ||||||