CBSE Guess > Papers > Question Papers > Class X > 2008 >Science > Science Theory
Or
(a) Give an example of a metal which (i) can be easily cut with a knife. (ii) is a liquid at room temperature.
(b) Write chemical equation for the reaction when (i) steam acts on red hot iron. (ii) zinc is added to iron (II) sulphate solution.
(c) What are alloys?
(d) Why are food cans coated with tin and not zinc?
Ans. (a) (i) Sodium metal can be easily cut with a knife.
(ii) Mercury is a liquid at room temperature.
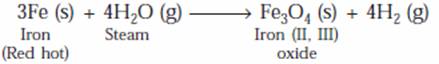
(b) (i) Red hot iron reacts with steam to form iron (II, III) oxide and hydrogen.
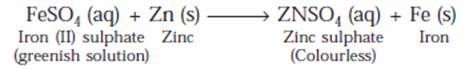
(ii) When Zinc is added to iron (II) sulphate solution, the greenish colour of iron (II) sulphate fades gradually due to the formation of colourless zinc sulphate solution and iron metal is deposited on zinc.
(c) The homogeneous mixture of two or more metals or metal and a nonmetal to improve the properties is called an alloy.
(d) Food cans are coated with tin and not zinc because zinc is a very reactive metal whereas tin is a less reactive metal. So the food contents like water and acids do not react with tin coating.
Section B
Q.17. How has the traditional use of wind energy been modified for our convenience?
Ans. The traditional use of wind energy has now been modified by the improvement in technology to generate electricity through windpowered generators.
Q.18. What is the minimum speed of wind to run a wind-mill to maintain the necessary speed of turbine in an electric generator?
Ans. The minimum speed of wind to run a wind-mill is 15 km/h to maintain the necessary speed of turbine in an electric generator.
Q. 19. Where do plants get each of the raw materials required for photosynthesis?
Ans. The raw materials for photosynthesis are :
(i) Carbon dioxide obtained from air.
(ii) Water obtained by the roots of the plants from the soil.
| Science 2008 Question Papers Class X | |||||||
| Delhi | Outside Delhi | Compartment Delhi | Compartment Outside Delhi | ||||
| All Sets (PDF) | Set 1 | Set 1 | |||||
| Set 2 | Set 2 | ||||||
| Set 3 | Set 3 | ||||||