CBSE Guess > Papers > Question Papers > Class X > 2008 >Science > Science Theory
Q. 11. The position of three elements A, B and C in the Periodic Table is shown below :
Ans.
| Group→ | I | II | III | IV | V | VI | VII | VIII |
| Period ↓ |
||||||||
| 1 | ||||||||
| 2 | B | |||||||
| 3 | A | C |
Giving reasons, explain the following :
(a) Element A is a metal.
(b) Element C has a larger size than element B.
(c) Element B has a valency of 3.
Ans. (a) Element A is a metal because it belongs to group I of the periodic table and thus has 1 valence electron, so it loses its one valence electron easily to form a monovalent positive ion.
(b) Element C has a larger size than element B because on moving from left to right in a period, the atomic number of elements increases which means that the number of protons and electrons in the atoms increases i.e. the extra electrons are being added to the same shell. Moreover, atomic size increases on moving down in a group.
(c) Element B has a valency of 3 because it loses 3 valence electrons to achieve the nearest inert gas configuration, so the valency is 3.
Q. 12. Observe the two test tubes A and B in the diagram given below and answer the following questions :
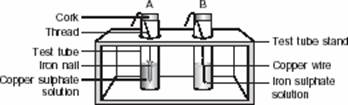
(a) In which test tube will the reaction take place?
(b) Write a balanced equation for the reaction.
(c) Name the type of reaction.
Ans.
- The reaction will take place in test tube ‘A’.
- CuSO4 (aq) + Fe (s) - FeSO4 (aq) + Cu (s) Iron is more reactive than Cu. Thus iron displaces copper from Copper Sulphate and forms FeSO4 and copper metal deposits on iron nail.
- It is a displacement reaction.
| Science 2008 Question Papers Class X | |||||||
| Delhi | Outside Delhi | Compartment Delhi | Compartment Outside Delhi | ||||
| All Sets (PDF) | Set 1 | Set 1 | |||||
| Set 2 | Set 2 | ||||||
| Set 3 | Set 3 | ||||||