CBSE Guess > Papers > Question Papers > Class X > 2008 >Science > Science Theory
Q. 8. A compound ‘X’ of sodium is commonly used in kitchen for making crispy pakoras. It is also used for curing acidity in the stomach. Identify‘X’. What is its chemical formula? State the reaction which takes place when it is heated during cooking.
Ans. (i) The compound ‘X’ is sodium hydrogen-carbonate commonly known as
baking soda.
(ii) The chemical formula of baking soda is NaHCO3.
(iii) Action of heat : When sodium hydrogen-carbonate is heated during the
cooking of food, it decomposes to give sodium carbonate with the
evolution of carbon-dioxide gas.
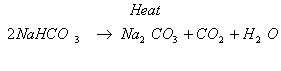 |
|||
| Sodium hydrogencarbonate (baking soda) |
carbonate Sodium | Carbon dioxide | Water |
Q. 9. Give reason of the following :
(a) Metals can be given different shapes according to our needs.
(b) Hydrogen is not evolved when a metal reacts with nitric acid.
Ans. (a) Metals can be given different shapes according to our needs because metals are malleable (i.e. metals can be hammered into thin sheets) and ductile (i.e. metals can be drawn into thin wires).
(b) Hydrogen is not evolved when a metal reacts with nitric acid because nitric acid is a strong oxidising agent. So, as soon as hydrogen gas is formed in the reaction between a metal and dilute nitric acid, the nitric acid oxidises this hydrogen to water.
Q. 10. Give reason of the following :
(a) Gold and silver are used to make jewellery.
(b) Carbonate and sulphide ores are usually converted into oxides prior
to reduction during the process of extraction.
Ans. (a) Gold and silver are used to make jewellery because .
(i) Gold and silver are the most malleable metals.
(ii) Gold and silver are the most ductile metals.
(iii) Gold and silver do not react with air, water and dil. acids. Therefore
these metals do not corrode when exposed to atmosphere.
(iv) Gold does not tarnish and retains its luster for years.
(b) Carbonate and sulphide ores are generally converted into oxides prior
to reduction during the process of extraction because it is easier to
obtain metals from their oxides than from carbonates or sulphides.
| Science 2008 Question Papers Class X | |||||||
| Delhi | Outside Delhi | Compartment Delhi | Compartment Outside Delhi | ||||
| All Sets (PDF) | Set 1 | Set 1 | |||||
| Set 2 | Set 2 | ||||||
| Set 3 | Set 3 | ||||||