CBSE Guess > Papers > Question Papers > Class X > 2008 >Science > Science Theory
Q. 10. The elements of the second period of the Periodic Table are given below :
Li Be B C N O F
(a) Give reason to explain why atomic radii decreases from Li to F.
(b) Identify the most (i) metallic and (ii) non-metallic element.
Ans. 2nd period → Li Be B C N O F
(a) Atomic radii decreases from Li to F because on moving from left to right, the atomic number of elements increases which means the number of protons and electrons in the atoms increases and extra electrons are being added to the same shell. Due to large positive charge on the nucleus, the electrons are pulled in more close to the nucleus and the size of the atom decreases.
(b) Most metallic element is Li, most non-metallic element is F. On moving from left to right in a periodic table the metallic character of elements decreases but the non-metallic character increases. Metals lose electrons and form positive ions so metals are electropositive whereas non-metals accept electrons and form negative ions, so nonmetals are electronegative.On moving from left to right in a period the nuclear charge (positive) increases as the number of protons increases, therefore the valence electrons are pulled in more strongly by the nucleus so the tendency of atoms to lose electrons decreases.
Q. 11. A convex lens has a focal length of 10 cm. At what distance from the lens should the object be placed so that it forms a real and inverted image 20 cm away from the lens? What would be the size of the image formed if the object is 2 cm high? With the help of a ray diagram show the formation of the image by the lens in this case.
Sol. Focal length of the convex lens, f = + 10 cm
Object distance, u = ? Image distance, v = + 20
(+ ve sign is because of the real nature of the image given)
Size of the object, h1 = + 2 cm
Size of the image, h2 = ?
According to lens formula
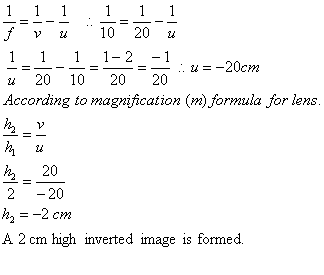
| Science 2008 Question Papers Class X | |||||||
| Delhi | Outside Delhi | Compartment Delhi | Compartment Outside Delhi | ||||
| All Sets (PDF) | Set 1 | Set 1 | |||||
| Set 2 | Set 2 | ||||||
| Set 3 | Set 3 | ||||||