CBSE Guess > Papers > Question Papers > Class X > 2008 >Science > Science Theory
Q. 12. (a) Why does an aqueous solution of an acid conduct electricity?
(b) How does the concentration of hydronium ions [H3O+] change when a solution of an acid is diluted? (c) Which has a higher pH value, a concentrated or dilute solution of hydrochloric acid?
(d) What would you observe on adding dilute hydrochloric acid to
(i) Solid sodium carbonate placed in a test tube? (ii) Zinc metal in a test tube?
Ans. (a) The aqueous solution of an acid conducts electricity due to the presence of charged particles (ions) in it.
(b) When a solution of an acid is diluted, the concentration of hydronium ions (H3O+) decreases because the Hydrogen ions (H+) given by an acid decrease on adding more water in the acid.
(c) The pH value of dilute solution of hydrochloric acid is more than concentrated HCl because the pH of a solution is inversely proportional to the concentration of hydrogen ions in it as dilute acids have less hydrogen ions than concentrated acids.
(d)
- When dil. HCl is added to solid sodium carbonate, sodium chloride, carbon dioxide and water are formed.
Na2CO3 (s) + 2HCl (aq) → 2NaCl (aq) + CO2 (g) + H2O (l)
When dil. HCl is added to the test tube containing solid sodium carbonate, a gas with brisk effervescence is produced which turns lime water milky (showing that it is carbon-dioxide gas).
- (ii) When dil. HCl is added to zinc metal in a test tube, gas bubbles are absorbed on the surface of zinc granules. If a burning candle is brought near the gas, the gas burns with a ‘pop’ sound (only hydrogen gas burns with a ‘pop’ sound).
Zn (s) + dil 2HCl (aq) → ZnCl2 (aq) + H2 (g)
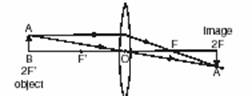
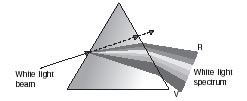
Q.13. What is meant by ‘Dispersion of White Light’? Draw a ray diagram to show dispersion of white light by a glass prism. Why do we get different colours of light?
Ans. The splitting up of white light into seven colours on passing through a transparent medium like a glass prism is called dispersion of light.
| Science 2008 Question Papers Class X | |||||||
| Delhi | Outside Delhi | Compartment Delhi | Compartment Outside Delhi | ||||
| All Sets (PDF) | Set 1 | Set 1 | |||||
| Set 2 | Set 2 | ||||||
| Set 3 | Set 3 | ||||||