- English
- Sociology
- Functional English
- Psychology
- Mathematics
- Philosopy
- Physics
- Computer Science
- Chemistry
- Entrepreneurship
- Biology
- Informatics Practices
- Geography
- Multimedia & Web Technology
- Economics
- Biotechnology
- Business Studies
- Physical Education
- Accountancy
- Fine Arts
- Political Science
- History
- Agriculture
CBSE Guess > Papers > Question Papers > Class XII > 2003 > Chemistry > Outside Delhi Set -II.
CHEMISTRY—2003 (Set II—Outside Delhi)
Note: Except for the following questions, all the remaining questions have been asked in Set I .
Q. 1. Write the composition of double-base propellant. 1
Q. 2. What is meant by inversion of sugar? 1
Q. 9. Name the radio active series which starts from Plutonium -241 and terminates at Bismuth-209. 1
Q. 10. Write a neutral molecule in which the central atom is  sp hybridized. 1
sp hybridized. 1
Q. 13. What is meant by specific conductivity of a solution?
The specific conductance of a 0.12 N solution of an electrolyte is 2.4 x 10 -2 S cm -1 . Calculate its equivalent conductance. 2
Q. 20. Write equations for the synthesis of the following: 2
(i) Glyptal
(ii) Neoprene
Q. 25. Illustrate the following with an example of reaction: 2
(i) Ambident nucleophile
(ii) Hinsberg test
Q. 27. Give appropriate reason for each of the following observations: 3
(i) Only higher members of Group 18 of the periodic table are expected to form compounds.
(ii) Fluorine is a stronger oxidising agent than chlorine, though fluorine has lower electron affinity than chlorine.
(ii) NO 2 readily forms a dimer, whereas CIO 2 does not.
Q. 28. Complete the following reactions: 3
(i) CH 3 - CH - CH 3 + PCI 5 -->
(ii) 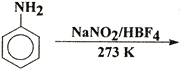
CH 3
|
(iii) CH 3 - C - X + NaOH -->
|
CH 3
Q. 29. What is meant by Vant's Hoff factor?
The osmotic pressure of a 0.0103 molar solution of an electrolyte is found to be 0.70 atm at 27 0 C. Calculate the Van't Hoff factor.
[R = 0.082 L atm-mol -1 K -1]
What conclusion do you draw about the molecular state of the solute in the solution?
Q. 32. Explain what is meant by dual nature of a particle in motion.
Show that the wavelength of a moving particle is related to its kinetic energy (E) as
 .
.
| Chemistry 2003 Question Papers Class XII | |||||||
| Delhi | Outside Delhi | Compartment Delhi | Compartment Outside Delhi | ||||
| Set I | Set I | Set I | Set I | ||||
| Set II | Set II | Set II | Set II | ||||
