![]()
CBSE Guess > Papers > Important Questions > Class XII > 2007 > Chemistry > Practice Paper 3
Practice Paper 3
- Write d-orbitals is envolved in dsp2 and sp3d hybridisation?
- Find out the no of waves made by bohr electron in one complete revolution in third orbit?
- Find the bond order of no-and co+ ions?
- Name one solid which has both schottky and frankel defect?
- The effective radius of an iron atom is 1.62 it has rock sall like structure . calculate its density {Fe =56 amu}?
- A mixture of chloro benzene and Bromo benzene is nearly an ideal solution . but a
mixture of chloroform and is not why? - The vapur pressure of a dil aqueasus solution of glucose [C6 H12 O6] is750mm of mercury at 373K. calculate [1] molality [2] mole farection of the solute3?
- If 20.0cm3 of 1.0M cacl3 and of 20m cacl2 are mixed ,what will be the resultant molarity?
- State and explain the third law of thrmodynamics?
- Calculate the free eneregy change of reactio
C[grphite] + O2[g] Co2
H = 300Kg mol-1 and S=3jK-1 mol-1
prodict whether the reaction is spontaneous or not at 300K? - Define electrochemical equivalent?
- Specific conductivity of a 12.N solution of an electrolyte is .024 determine its equivalent conductivity?
- Calculate the max. work that can be obtained from the cell 2n/2 Given E
- Define molealerity of a reaction
For a 1st odrer reaction the ratio the time taken to complete three fourth of the reaction and the time taken to complete half of reaction.? - Define cataphoresis?
- Explain cleansing action of soap
Arrange [I] NH3,PH3, increasing order of basic strength. [ii] H2 is decresing order of boiling point. - Why meta phosphoric acid is………..
- NCL3 gets readily ………………while NFf3 does not why
- Why BF3 is weaker lewis acid than Bcl3?
- Explain the following given reason.
(I) Compounds of transition metals are after colon red. ?
(II) Transition elements exhibit variable oridation state.?
(III) Transition elements have high melting and B.P.?
(IV) Ticl3coloured while Ticl4 is colour less - (I) Give the IUPAC name of
[Pt(SCN)(NH3)3](SCN)
(II) Write the formula of the WilkinsinsCatalyst? - Product the structure and hybridisation of in ...............
- Ten grams of an X active radioactive isotope are .............................is a container .In one ........ the he gas collecetd at ....is 11.2 Calculate the half life of radioisotope?
- What are ....................and........................
- [a] Give the IUPAC names of

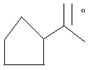
[b] why phenol is more reactive than benzene towards electrophelic substitution reaction
[c] explain .............. rule - How will you comment -
Acetophenon to benzoic acid? - why cyclohexylamine is a stronger base then .............?
- How will you prepare terylene or ............?
- How do amino acids form proteins ?
- Distinguish between the terms antigenes and antibodees?
- What are the consituents of starch?
- Give an example of sulpha drug?
- Define [i] Antipyreties [ii] A................ with example?
- Atomic Structure and Chemical Bonding
- Solid State
- Solutions
- Thermodynamics
- Electrochemistry
- Chemical Kinetics
- P Block Elements
- D and F Block Elements
- Nuclear Chemistry
- Stereo Chemistry
- Organic Functional Group I
- Organic Functional Group II and III
- Surface Chemistry
- Chemistry In Every Day Life
- Polymers
- Practice Paper No.1
- Practice Paper No.2
- Practice Paper No.3
- Practice Paper No.4
